Limestone is composed almost entirely of calcite and will produce a vigorous fizz with a drop of hydrochloric acid.
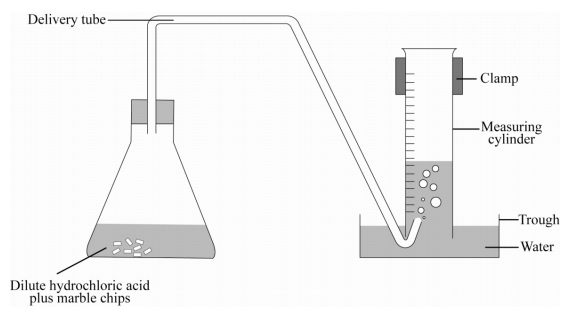
Marble in dilute hcl.
When the reaction was complete 1 1 2 0 c m 3 of o 2 was obtained s t p.
Marble is found in various places around the world including india greece spain turkey egypt italy and the united states of america.
10 gram of a piece of marble was put into excess of dilute hcl acid.
2 hcl aq caco3 s cacl2 aq h2o l co2 g if you are talking about a marble counter top then i would advise against adding dilute hcl to that surface that because you will ruin it.
What change would be observed in lime water.
The percentage of c a c o 3 in the marble is.
Marble reaction with hydrochloric acid drop a small amount of dilute hydrochloric acid on an area of your sample that has been scratched by a nail.
Pieces of marble are thrown into hydro chloric acid.
Calcium carbonate is dissolved by hydrochloric acid thereby forming gaseous carbon dioxide.
Some rocks contain carbonate minerals and the acid test can be used to help identify them.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
The reaction takes place spontaneously.
When you mix a strong acid like hcl with any carbonate you will have a chemical reaction in which co2 gas is released it will fizz.
The evolved gas was then passed through lime water.
It will produce a very weak fizz when a drop of cold hydrochloric.
A student dropped a few pieces of marble in dilute hydrochloric acid contained in a test tube.
With the help of balanced chemical equations for all the changes explain the observations.
Limestone dolostone and marble.
Asked oct 28 2017 in class x science by priya12 12 631 points.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
The acid test on rocks.
The chemical equation for the reaction is as follows caco3 2hcl cacl2 h2o co2 carbon dioxide gas turns lime water milky due to the formation of calcium carbonate.
Marble is chemically calcium carbonate.
When dilute hcl is added to calcium carbonate it forms calcium chloride water and carbon dioxide.
A student dropped few pieces of marble in dilute hydrochloric acid contained in a test tube.
What will happen if an excess of gas is passed through lime water.
While marble can appear superficially similar to quartzite a piece of marble will be able to be scratched by a metal blade and marble will fizz on contact with dilute hydrochloric acid.
The combined reactants have a higher chemical potential than the combined products i e.